Evaluation of the Safety and Efficacy of Neurotrophic factor therapy in Ischemic Stroke
Cerebral stroke, also known as "stroke" or "cerebrovascular accident" (CVA), is an acute cerebrovascular disease characterized by sudden rupture of blood vessels in the brain or obstruction of blood flow to the brain, resulting in damage to brain tissue. It includes both ischemic and hemorrhagic strokes. Stroke is the second leading cause of death globally, with high mortality and disability rates in adults.
Ischemic Stroke (IS) refers to the localized ischemic necrosis or softening of brain tissue caused by cerebrovascular circulation disorders, ischemia, and hypoxia. Due to its high incidence, prevalence, mortality, and disability rate, it has become one of the major causes of the global disease burden. According to statistics, approximately 15 million people worldwide suffer from ischemic stroke each year, with 5 million deaths and 5 million individuals experiencing long-term disabilities.
The Mechanism of Stem Cell Therapy for Ischemic Stroke
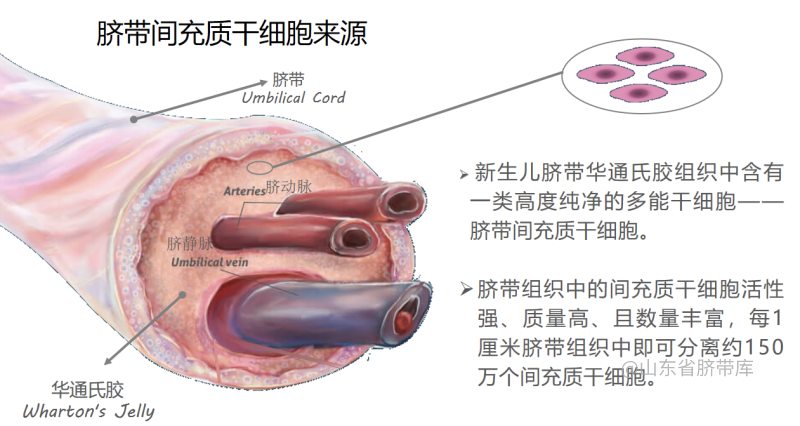
Mesenchymal Stem Cells (MSCs) are a type of multipotent stem cells that can be isolated from various tissues, including bone marrow, adipose tissue, and umbilical cord blood. Due to their pluripotent properties, they can differentiate into osteoblasts, chondrocytes, adipocytes, and even neurons. Currently, MSC transplantation has emerged as a novel therapeutic approach for ischemic diseases, including stroke, coronary artery disease, and peripheral artery disease. Multiple studies have shown that the excellent neuroprotective effect of MSCs is a key factor in treating ischemic stroke.
Current research reports indicate that MSC mainly promotes the repair of neural tissue damage, restoration of neural function, reduction of cerebral infarction size, and promotion of neurogenesis and angiogenesis through paracrine effects, immunomodulatory effects, and anti-apoptotic effects, thus playing a therapeutic role in ischemic stroke.
Paracrine effect
MSC's paracrine factors, including exosomes and extracellular vesicles, are the main contributors to their therapeutic effects. In the area of cerebral stroke injury, MSCs can induce the expression of various growth factors, including brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), stromal cell-derived factor 1 (SDF-1), basic fibroblast growth factor (bFGF), glial cell-derived neurotrophic factor, and hepatocyte growth factor, among others. These factors help reduce the area of cerebral infarction and restore neural function, thereby improving cerebral stroke injuries.
The immunomodulatory effect
Research has found that MSCs also have a dual regulatory function on central immune responses after stroke: On the one hand, they can significantly increase the number of B cells, NK cells, and T cells in brain tissue, exerting an immune-promoting effect. On the other hand, high levels of inflammatory factors can activate the immunosuppressive function of MSCs, thereby exerting a dual inhibitory effect on lymphocyte proliferation and inflammatory factor production, inducing an anti-inflammatory phenotype.
Regulation of cell apoptosis
Cell apoptosis is an important mechanism of neuronal injury in ischemic stroke, and MSCs can inhibit neuronal apoptosis, exerting a neuroprotective effect. MSC therapy can also alleviate ischemic stroke-induced astrocyte necrosis, and moreover, hypoxia-preconditioned MSCs demonstrate better therapeutic effects in reducing cell necrosis induced by cerebral hemorrhage.
The clinical evaluation of stem cell therapy for ischemic stroke.
Transplantation of mesenchymal stem cells (MSCs) for the treatment of ischemic stroke has shown promising results in preclinical studies. Researchers conducted a meta-analysis of 20 clinical trials involving a total of 1127 patients, including 573 in the experimental group (treated with MSCs) and 554 in the control group (conventional treatment). Compared to conventional treatment, MSC transplantation significantly reduced the National Institutes of Health Stroke Scale (NIHSS) scores at 1, 3, 6 months, and 1 year post-treatment. It also significantly improved the activities of daily living index at 1 and 6 months, as well as motor function and functional independence scores at 1, 3, and 6 months.
The main evaluation indicators include: (1) National Institutes of Health Stroke Scale (NIHSS) score; (2) Barthel Index (BI); (3) Fugl-Meyer Assessment (FMA) score; (4) Functional Independence Measure (FIM) score; and (5) Modified Rankin Scale (mRS) score.
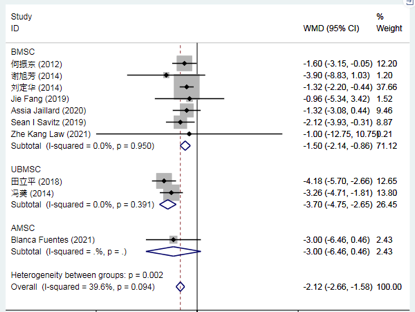
Compared to conventional treatment, mesenchymal stem cell (MSC) therapy for stroke significantly reduces the NIH Stroke Scale (NIHSS) score at 3 months.
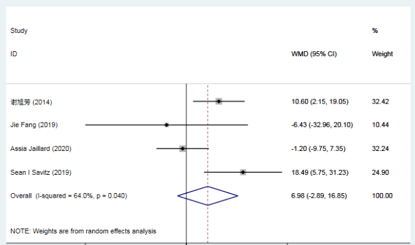
Compared to conventional treatment, there was no significant difference in the Barthel Index (BI) score at 3 months with mesenchymal stem cell (MSC) therapy for stroke.
Compared to conventional treatment, mesenchymal stem cell (MSC) therapy for stroke significantly improved the Functional Movement Assessment (FMA) score at 3 months.
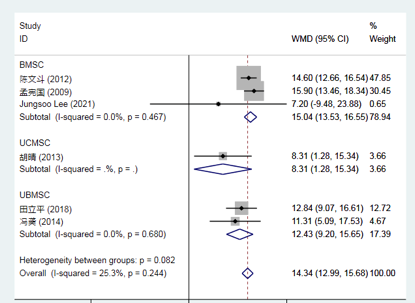
Compared to conventional treatment, mesenchymal stem cell (MSC) therapy for stroke significantly improved the Functional Independence Measure (FIM) score at 3 months.
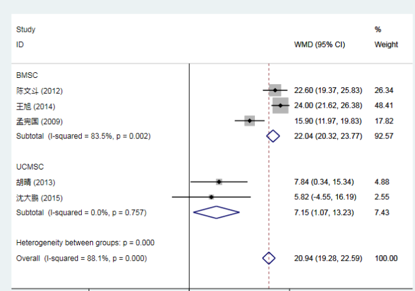
Compared to conventional treatment, mesenchymal stem cell (MSC) therapy for stroke showed no significant difference in the Modified Rankin Scale (mRS) score at 3 months.
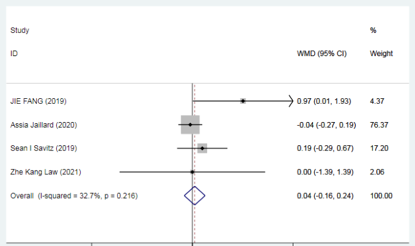
Taken together, a large body of preclinical studies demonstrates the feasibility and effectiveness of mesenchymal stem cell (MSC) transplantation therapy in the treatment of ischemic stroke, which to some extent improves neurological deficits, motor function, and activities of daily living in patients with ischemic stroke.
Future and Prospects
In recent years, from the simple differentiation and transformation cognition of stem cell research to the continuous deepening and expansion of the diverse mechanisms of MSC research, the development of the field of stem cell research has become mature and innovative. Currently, not only have animal experiments confirmed the therapeutic effects of stem cells, but also the feasibility of MSC treatment for ischemic stroke has been proposed. In numerous clinical trials, MSC therapy for ischemic stroke is steadily advancing.
As of recently, there have been 101 clinical trials registered in the US clinical trial database for stem cell therapy for stroke, with 25 studies related to MSC treatment for ischemic stroke. Clinical trials have demonstrated that MSCs have a significant improvement in neurological function for ischemic stroke patients, confirming their effectiveness and long-term safety.
In the future, there will be more medical breakthroughs involving MSCs that will be translated into clinical applications, contributing to the healthcare industry and safeguarding the health and well-being of people worldwide.